Electron Activity in Chemical Reactions Lead acid Cell Battery
Lead-acid batteries are one of the most common secondary batteries, used primarily for storing large cell potential. These are commonly found in automobile engines. Its advantages include low cost, high voltage and large storage of cell potential; and disadvantages include heavy mass, incompetence under low-temperatures, and inability to.
Electrochemistry
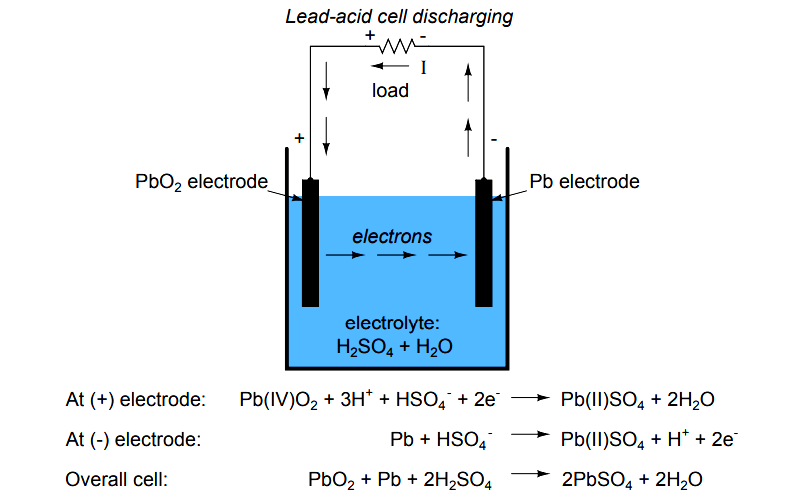
The lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. The following half-cell reactions take place inside the cell during discharge: At the anode: Pb + HSO4- → PbSO4 + H+ + 2e-. At the cathode: PbO2 + 3H+ + HSO4- + 2e- → PbSO4 + 2H2O. Overall: Pb + PbO2 +2H2SO4 → 2PbSO4 + 2H2O.
What is a lead acid battery? Knowledge Base
Context 1. number as such also depends on the dynamics of changes in the load of the analysed battery. The electric diagram of the discussed n-order model of a single cell of the.
6.10.1 Lead/acid batteries Engineering LibreTexts
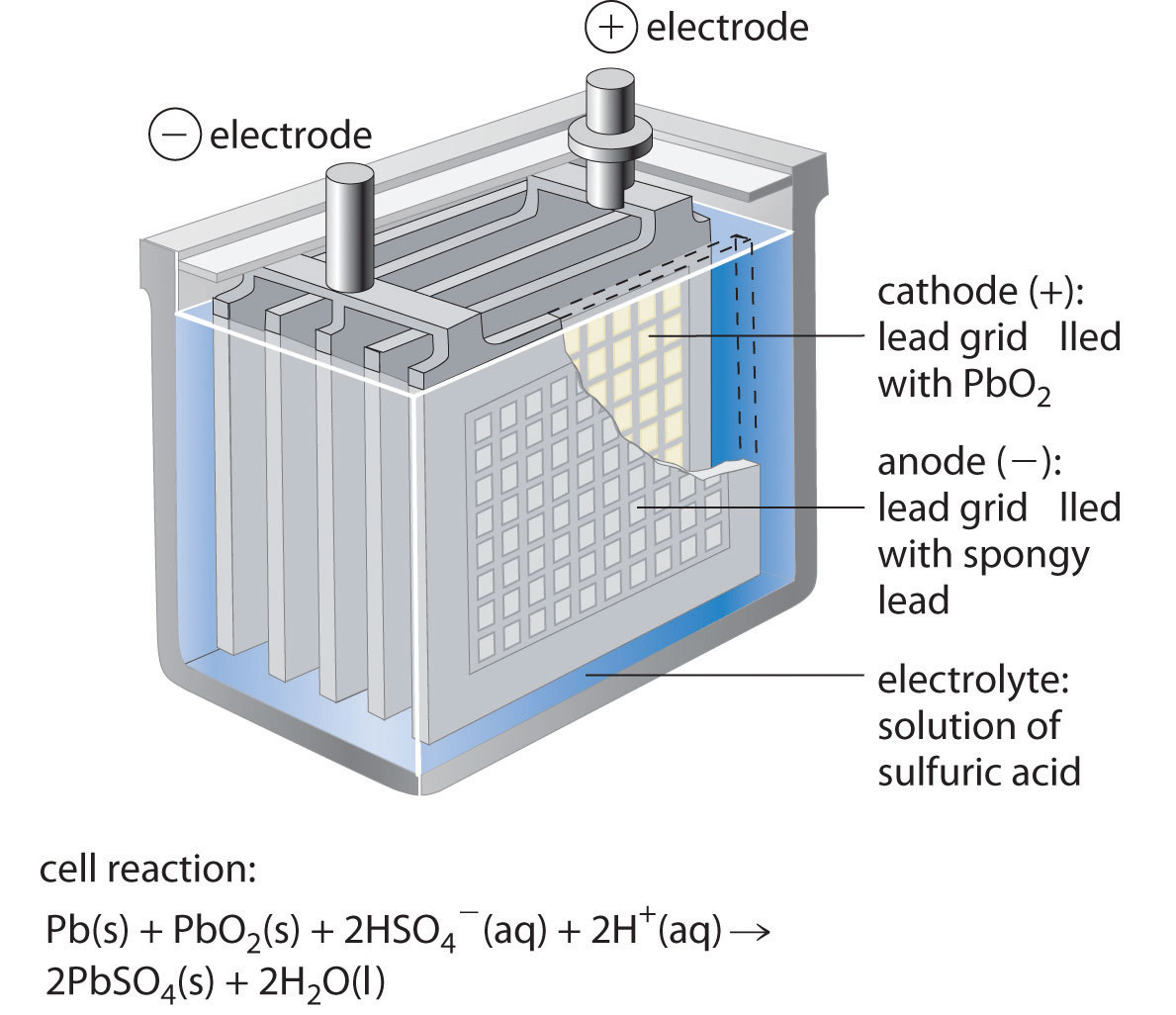
Figure \(\PageIndex{3}\): One Cell of a Lead-Acid Battery. The anodes in each cell of a rechargeable battery are plates or grids of lead containing spongy lead metal, while the cathodes are similar grids containing powdered lead dioxide (PbO 2). The electrolyte is an aqueous solution of sulfuric acid. The value of E° for such a cell is about.

How lead acid battery works Working principle animation YouTube
The lead-acid battery is the most commonly used type of storage battery and is well-known for its application in automobiles. The battery is made up of several cells, each of which consists of lead plates immersed in an electrolyte of dilute sulfuric acid. The voltage per cell is typically 2 V to 2.2 V.

Engineering Photos,Videos and Articels (Engineering Search Engine) CHAPTER 2 PRIMARY AND
Lead-Acid Battery. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. The supplying of energy to and external resistance discharges the battery. Lead-acid batteries. Index. DC Circuits. Batteries. HyperPhysics ***** Electricity and Magnetism. Go Back.

PPT The Lead Acid Electric Battery PowerPoint Presentation, free download ID1810673
A lead-acid battery cell consists of a positive electrode made of lead dioxide (PbO 2) and a negative electrode made of porous metallic lead (Pb), both of which are immersed in a sulfuric acid (H 2 SO 4) water solution. This solution forms an electrolyte with free (H+ and SO42-) ions. Chemical reactions take place at the electrodes:
All about Batteries Electrical A2Z
In the charging process we have to pass a charging current through the cell in the opposite direction to that of the discharging current. The electrical energy is stored in the form of chemical form, when the charging current is passed. lead acid battery cells are capable of producing a large amount of energy. Construction of Lead Acid Battery.

a Characteristics curve of one lead acid cell at different discharge... Download Scientific
A lead acid battery consists of a negative electrode made of spongy or porous lead. The lead is porous to facilitate the formation and dissolution of lead. The positive electrode consists of lead oxide. Both electrodes are immersed in a electrolytic solution of sulfuric acid and water.

/1 Typical structure of a lead acid battery Source Chemistry Libre... Download Scientific
difference (usually measured in volts) is commonly referred to as the voltage of the cell or battery. A single lead-acid cell can develop a maximum potential difference of about 2 V under load. A completely discharged lead-acid cell has a potential difference of about 1.75 V, depending on the rate of discharge. Capacity and Battery Ratings

Geometry of a leadacid cell. (a) macroscopic shape and dimensions; (b)... Download Scientific
The most common type of heavy duty rechargeable cell is the familiar lead-acid accumulator ('car battery') found in most combustion-engined vehicles. This experiment can be used as a class practical or demonstration. Students learn how to construct a simple lead-acid cell consisting of strips of lead and an electrolyte of dilute sulfuric acid.

Primary cell electronics Britannica
The open circuit voltage of this battery is. VO = (2:041. 0:12pH) V: (22) To see the range of conditions in which this battery can e ectively operate, we consider the Nernst equation for various possible reactions: Lecture 9: Fuel cells and lead-acid batteries 10.626 (2011) Bazant. Lead / sulfuric acid (half-cell) reactions:

Basics of VRLA Type of Batteries
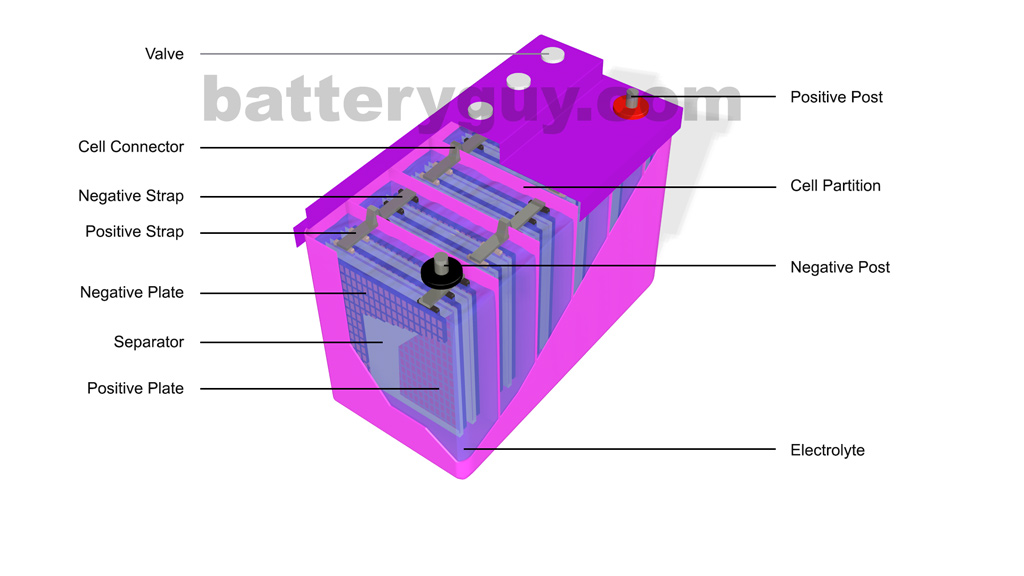
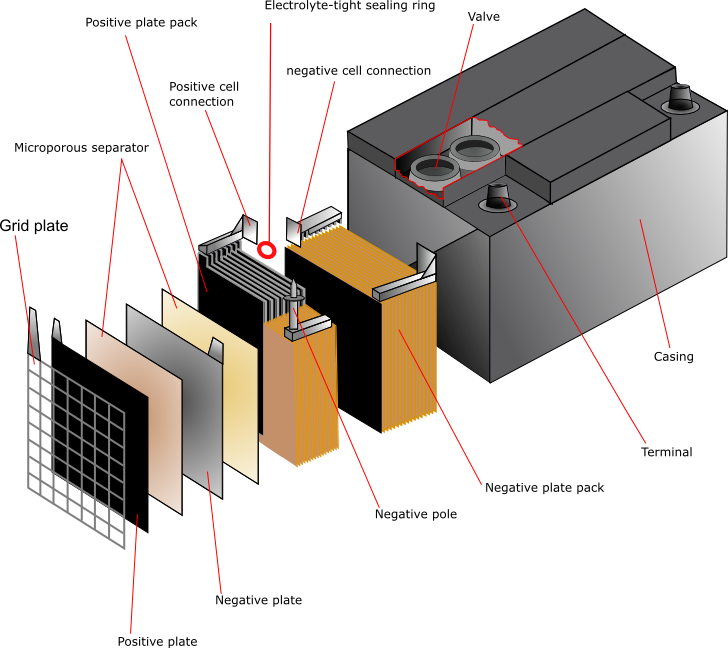
Lead acid Cell Working Principle: Construction of Lead-acid battery or lead-acid storage battery: Lead-acid battery Container: Lead-Acid battery Plates: Positive and Negative plates: Separators: Electrolyte: Element: Container, Cell covers, and vent plugs: Battery terminals: Cell connectors: Charging of lead acid battery:
Schematics of leadacid battery cells Download Scientific Diagram
This corresponds that lead acid cells possess a high amount of power to weight proportions.. The lead acid battery diagram is. Lead Acid Battery Diagram Container. This container part is constructed with ebonite, lead-coated wood, glass, hard rubber made of the bituminous element, ceramic materials, or forged plastic which are placed on the.

Lead acid battery, Construction and, Working, and Charging
Plante Process In this process two sheets of lead are taken and immersed in dilute H 2 SO 4. When an current is passed into this lead acid cell from an external supply, then due to electrolysis, hydrogen and oxygen are evolved.

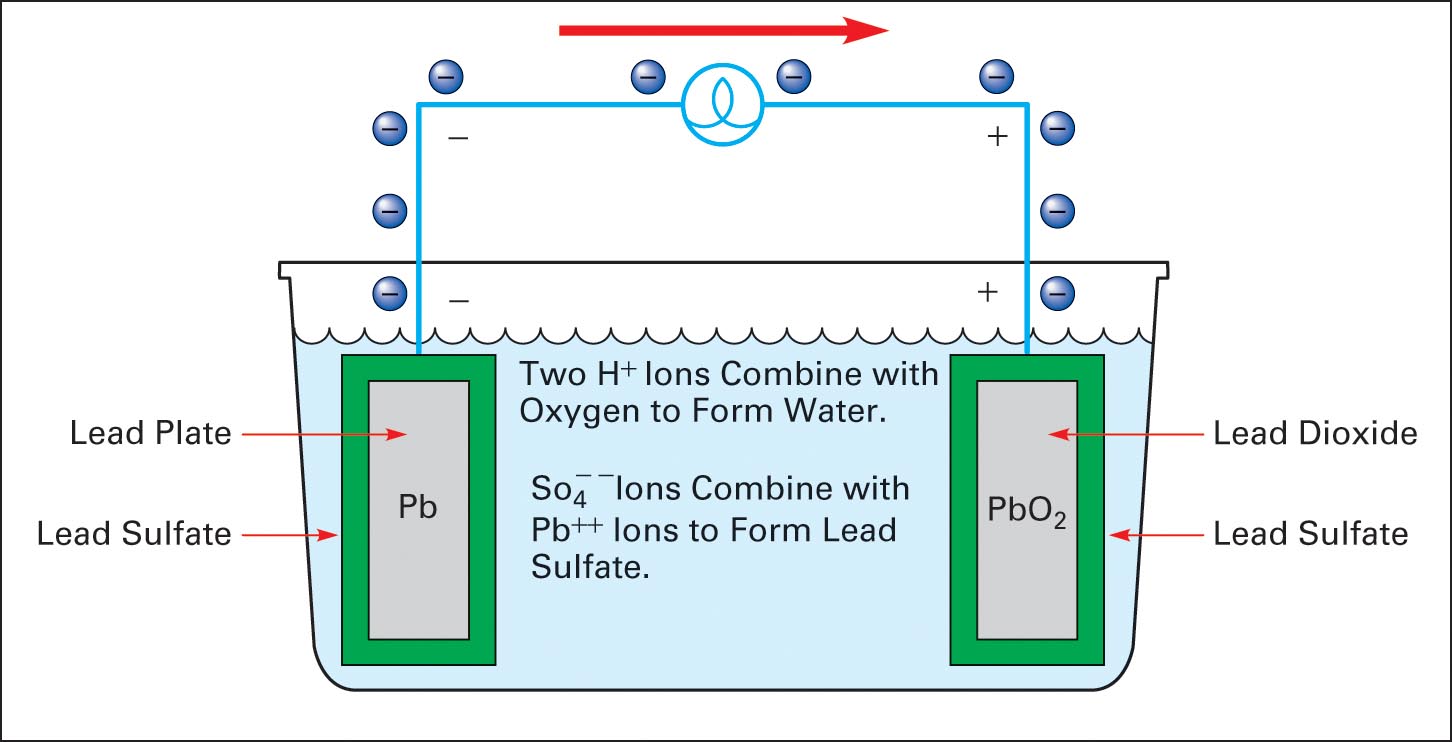
How a lead acid cell works. Electrical Academia
Figure \(\PageIndex{3}\) A diagram of a cross section of a dry cell battery is shown. The overall shape of the cell is cylindrical. The lateral surface of the cylinder, indicated as a thin red line, is labeled "zinc can (electrode).". The lead acid battery (Figure \(\PageIndex{5}\)) is the type of secondary battery used in your.